scott@vtx-cpd.com
Forum Replies Created
-
AuthorPosts
-
Replying to Sarah Keir 14/08/2025 - 09:37
Thanks Sarah, totally agree, DCM is a real consideration at this stage and something we’ll need to keep on the radar going forward, I must admit I often forget this sort of thing!. Look forward to your thoughts on the liver side when you’re back.
Best,
Scott 🙂
Replying to Victoria R. 14/08/2025 - 09:40
Hi Tori,
I’d say I’m the same, I haven’t really noticed a strong pattern either. If anything, I’ve sometimes thought the occasional GI signs might be more about the timing with food rather than the drug itself, since like you, I usually give it alongside a meal. Many of the patients I end up using it in have chronic enteropathy, so they’re already showing GI signs to begin with, which makes it tricky to separate out. I might start paying closer attention to see if there’s any consistent trend.
Scott 🙂
Replying to Victoria R. 14/08/2025 - 10:09
Hi Tori,
That’s really interesting, thanks for sharing. I must admit I don’t use antihistamines in many of my own patients. I’m working in Canada now, and they love Benadryl here! I do tend to reach for antihistamines more often in cases of lymphocytic–plasmacytic rhinitis, usually alongside an NSAID. That said, I’m not sure how effective I actually find them, and I often end up reaching for steroids instead.
Scott 🙂
Replying to Victoria R. 14/08/2025 - 10:11
Hahaha!
Met too! I think I just need to get better organised and start doing it!
Scott 🙂
Replying to Elizabeth Murch 14/08/2025 - 13:46
Radiography report:
Abdomen
Serosal detail: Within normal limits for both peritoneal and retroperitoneal spaces.
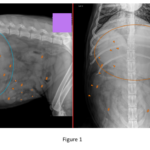
Foreign material: Multiple, thin, linear metallic opacities within the peritoneal cavity and possibly in the small intestinal lumen (Figure 1, arrowheads) – likely incidental barbecue brush bristles.
Stomach: Moderately to markedly distended with homogeneous fluid/soft tissue opaque material and gas (Figure 1, ovals). Gas redistributes normally between views. The pylorus appears empty in the left lateral view (Figure 1, arrow).
Small intestine: Descending duodenum and several other loops are overdistended with homogeneous fluid/soft tissue opaque material and scant gas, with a stacked appearance (Figure 1, #). Other loops are empty (**Figure 1, ***).
Colon: Mildly distended with formed soft tissue opaque fecal material and gas. Cecum not identified.
Other abdominal structures: Liver, spleen, visible renal margins, urinary bladder, and musculoskeletal structures are within normal limits.
Thorax
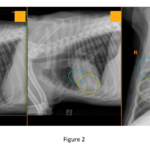
Pulmonary pattern: Alveolar patterns are present in the ventral right middle lung lobe, caudal subsegment of the left cranial lung lobe, and caudoventral aspect of the right cranial lung lobe (Figure 2, ovals).
Other thoracic structures: Trachea normal in diameter and position, cardiac silhouette, pulmonary vessels, and caudal vena cava are small. No pleural abnormalities. Musculoskeletal structures within normal limits.
Conclusions
Abdomen: Two populations of small intestine most consistent with a mechanical ileus. Metallic peritoneal/intestinal foreign bodies (barbecue brush bristles) likely incidental.
Thorax: Aspiration pneumonia in the right cranial, right middle, and left cranial lung lobes.
Additional Comments
Findings are most consistent with a small intestinal obstruction, which likely explains the clinical signs. Cause is not identified, but given chronicity, possibilities include:
Intestinal mass
Chronic, non-mineral foreign body
Intussusception
Abdominal ultrasound is recommended to determine the cause.
Replying to Elizabeth Murch 14/08/2025 - 13:46
Hi Elizabeth,
Lovely to hear from you, I hope all is well.
I agree those are the two main possibilities we need to work through. Given the chronicity but recent deterioration, a more detailed abdominal ultrasound would definitely be a good next step. It could help us identify whether there is a focal obstructive lesion (mass, intussusception, foreign body) or if the changes are more in keeping with a functional ileus from inflammatory or infiltrative disease.
If the ultrasound is inconclusive or if we need to better evaluate mural changes and surrounding structures, a CT scan would be the next logical step. CT would also give us the added benefit of staging if we were to find a mass, and could help clarify any concurrent thoracic changes.
Let me share some details from the radiography report!
Scott 🙂
Replying to Julia Biernat 12/08/2025 - 15:19
No problem!
Let me know if you have any other questions.
Scott 🙂
Felipe!
Thank you again for another brilliant video!
Scott 🙂
Replying to valerie dromey 04/08/2025 - 22:46
It was an interesting case…
Sad outcome with this one however.
Have a great eek.
Scott 🙂
Replying to Ingrid T. 05/08/2025 - 09:21
Ingrid!
Thank you so much again for working with us and delivering such a brilliant course!
I hope all is well.
Scott 🙂
Hey Shannon.
I hope you are well. I am sorry again about the delay with this lesson.
I would love to hear how you are getting on with the course? Any feedback would be really appreciated.
Scott 🙂
Felipe!
Thank you for sharing another brilliant video!
Scott 🙂
Replying to Iulia M. 07/08/2025 - 17:27
Hi Lulia,
Chlorphenamine can certainly be effective, but its shorter duration of action and higher sedation potential can be limiting. Cetirizine generally allows once-daily dosing, causes less sedation, and has some evidence in dogs, mainly small pharmacokinetic and clinical studies—suggesting it may help control allergic symptoms, particularly in atopic dermatitis. Direct head-to-head comparisons in veterinary patients are lacking, so choice is still based largely on individual response and tolerability. In my experience, cetirizine is worth trialling if chlorphenamine gives only partial control or causes unwanted sedation.
Hope you have a great week.
Scott 🙂
Replying to Julia Biernat 11/08/2025 - 09:31
Hi Julia,
Great question!
In most situations where we are looking to support glutathione production in liver disease, S‐adenosylmethionine (SAMe) is my first choice if the patient can tolerate oral tablets. SAMe is a physiological precursor for glutathione and has broader hepatoprotective effects including membrane stabilisation and modulation of oxidative injury. When given at appropriate doses on an empty stomach its bioavailability is good and it is generally well tolerated.
N acetylcysteine (NAC) on the other hand is most useful when oral SAMe is not feasible for example in a hospitalised or anorexic patient or one with severe nausea because it can be given IV (or orally though that is less common outside toxicity protocols). NAC is a great option in sick animals not tolerating oral medication. It acts as a glutathione precursor by providing cysteine but it also has direct antioxidant and mucolytic properties. In most chronic or subacute liver cases NAC and SAMe are not used together as standard because they do not have proven synergistic effects in that setting. You are essentially trying to achieve the same endpoint, glutathione replenishment, via two different routes.
The main exception is paracetamol (acetaminophen) toxicity where NAC is the drug of choice and should be given as early as possible ideally within hours of ingestion. In that context NAC is much more than a glutathione precursor. It also directly detoxifies NAPQI (the toxic metabolite), supports mitochondrial function and limits oxidative injury to red cells and hepatocytes. SAMe can be added later in paracetamol cases once the patient can tolerate oral medication but the acute life saving antidote is NAC.
So in summary:
• Chronic or subacute hepatopathies → use SAMe if tolerated, NAC if oral dosing is not possible.
• Not routinely combined for liver support as there is no clear evidence of synergy.
• Paracetamol toxicity → NAC is the priority and should be given immediately, SAMe may be added later as supportive therapy.I hope that helps! Have a lovely week.
Scott 🙂
Replying to Julia Biernat 11/08/2025 - 09:45
Hi Julia,
Thank you so much for joining the course and thank you for your question!
I think this is an area where the use of PPIs in liver disease is often a bit over-generalised.
In most stable chronic hepatopathies, I don’t use PPIs routinely. My main indications would be when I have reasonable clinical suspicion or confirmation of an upper GI bleed — for example, haematemesis, melaena, or gastric/duodenal erosions or ulceration seen on endoscopy — and I think the bleed is likely due to a combination of portal hypertension–related mucosal congestion, secondary erosive gastritis, or concurrent drug effects (e.g., corticosteroids, NSAIDs). I might also consider a PPI if there’s marked hyperacidity suspected in a case with concurrent GI disease or after a known ulcerogenic insult.
If I have a patient with evidence of bleeding but significantly prolonged clotting times, then yes, I’d generally want to correct the coagulopathy before performing any invasive diagnostics and ideally before starting acid suppression. This is particularly relevant if you think the bleed is related to mucosal friability secondary to hypocoagulability rather than an acid-mediated ulcer. In those cases, fresh frozen plasma (FFP) is my first step, as it provides both clotting factors and, in some cases, volume support for hypoalbuminaemic patients.
In terms of how often I see GI bleeding purely due to clotting factor abnormalities, I’d say it’s uncommon as the sole cause in liver disease. When it does occur, it’s usually in the context of advanced decompensation, either severe synthetic failure with markedly prolonged PT/aPTT or DIC. In those situations, GI bleeding is often part of a broader haemorrhagic tendency affecting multiple sites (e.g., petechiation, ecchymoses, haematuria). I do occasionally see bleeding in patients with severe thrombocytopaenia — whether from DIC, immune-mediated mechanisms, or bone marrow suppression — but in my experience, portal hypertensive gastropathy or erosive gastritis from systemic illness are more frequent contributors in liver patients.
For additional context, the ACVIM consensus on acid suppressant use notes that although hepatic disease has been associated with gastroduodenal ulceration (GUE) in dogs, evidence that it is a direct cause is scarce, and the prevalence of upper GI bleeding in dogs and cats with hepatic disease has not been established. The pathogenesis is uncertain. In humans, altered mucosal blood flow from portal hypertension (hypertensive gastropathy) is the most common cause for GI bleeding, while in dogs, decreased hepatic degradation of gastrin with subsequent hyperacidity has been proposed — though experimental data do not support hypergastrinemia as a major factor. Experimental bile duct ligation can produce ulceration in dogs. In people, gastric acid suppression generally does not reduce bleeding from portal hypertensive gastropathy because these patients already tend to have hypochlorhydria, although PPIs may indirectly help by raising gastric pH enough to stabilise clots. There is one retrospective canine study in which implementation of lifelong PPI therapy around the time of intrahepatic shunt closure was associated with a lower rate of death from GI haemorrhage, but the underlying mechanism was unclear and may not have been related to the hepatic dysfunction itself.
Overall, the consensus opinion is that there is weak evidence to support prophylactic acid suppression in dogs and cats with hepatic disease in the absence of clinical or endoscopic evidence of GI bleeding. My own approach mirrors this, I reserve PPIs for patients with documented or strongly suspected ulceration or erosive gastritis, rather than using them as blanket prophylaxis in all liver cases.
I hope you have a wonderful week!
Scott 🙂
-
AuthorPosts